HOW TO TAKE FIASP®
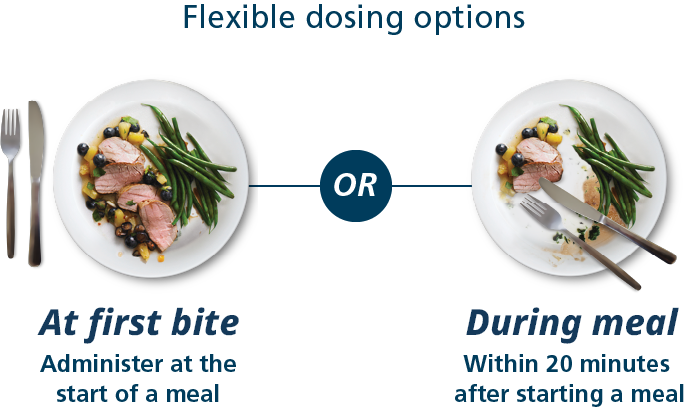
Fiasp® offers adjustable dose timing
Fiasp® can be taken at the start of a meal or within 20 minutes after starting a meal, whereas NovoLog® (insulin aspart) injection 100 U/mL must be taken 5 to 10 minutes before a meal. This range gives you flexibility when taking your mealtime insulin. Talk with your health care provider to find the appropriate time to take Fiasp® based on your individual needs and goals.
NovoLog®

Fiasp®
With Fiasp®, you have options for insulin delivery
Fiasp® can be administered via insulin pens, a vial and syringe, or an insulin pump or automated insulin delivery system like the iLet Bionic Pancreas.a Your diabetes care team will help determine the right option for you.
aRefer to the insulin pump or automated delivery system user manual to see if Fiasp® can be used. Use in accordance with the insulin pump or automated insulin delivery system instructions for use.
Expand to learn about the delivery options below
- Prefilled insulin pen
- Accurate dosing up to 80 units
- No push-button extension
- End-of-dose click, letting you know that a dose has been administeredb
bAfter dose counter has returned to 0, keep needle in skin for 6 seconds before the needle is removed. If you do not, you may not have received your full dose and you should check your blood sugar levels more often because you may need more insulin. You may or may not hear an audible click at end of dose.
Details on NovoPen Echo®
- Adjustable half-unit dosing from 0.5 to 30 units of insulin
- Automatically records the last dose and approximate time since your last injection
- Short button travel reduces injection movement
- You’ll know when to change the cartridge because Fiasp® NovoPen Echo® doesn’t allow you to dial more units than are left
- The pen injector is compatible with Lilly Humalog® U-100 3.0 mL cartridges, Novo Nordisk NovoLog® U-100 3.0 mL cartridges, Novo Nordisk Fiasp® U-100 3.0 mL cartridges, and single-use detachable and disposable pen needles (not included)
Save on your NovoPen Echo® with a savings card.
Get savings at NovoPenEchoSavings.com or call 1-888-910-1269 to sign up.d
Details on Fiasp® PenFill® cartridge
- PenFill® is a disposable cartridge that is loaded into your NovoPen Echo®, allowing for half-unit dosing
- Save on your PenFill® cartridges with a Fiasp® Savings Card. Get savings at FiaspSavings.com.
cPenFill® cartridges require a prescription and are sold separately.
An insulin pump is a small, programmable, battery-operated device that delivers a steady, measured amount of insulin under your skin.
Fiasp® in an insulin pump offers:
- Heat stability at normal body temperatures (up to 98.6°F)
- Storage in the pump reservoir for up to 6 days before being changed
If you use an insulin pump, it’s a good idea to have a backup source for insulin. Ask your diabetes care team if Fiasp® FlexTouch® or NovoPen Echo® could be a good option for you.
Refer to the insulin infusion pump user manual to see if Fiasp® can be used. Use in accordance with the insulin pump’s instructions for use.
- 1.6 mL single-patient-use PumpCart® cartridges are ready for use directly in compatible insulin pumps
- Check the insulin pump manual to see if PumpCart® can be used with your pump
Use in accordance with the insulin pump’s instructions for use.
If you use an insulin pump, it’s a good idea to have a backup source for insulin. Ask your diabetes care team if Fiasp® FlexTouch® or NovoPen Echo® could be a good option for you.
Fiasp® PumpCart® can be used in the iLet Bionic Pancreas
The iLet Bionic Pancreas is the only FDA-cleared automated insulin delivery system that makes 100% of your insulin dosing decisions. No more carb counting,* correction factors, or carb ratios.
*User must be carb aware.
iLet Bionic Pancreas is a registered trademark of Beta Bionics, Inc, and is used with permission.

STORAGE AND TRAVEL
Storage conditions for Fiasp® FlexTouch®, PenFill® and PumpCart® cartridges, and the viale-g
| Prior to first use (unopened) | Temperature | Use time | ||
| FlexTouch®, PenFill®, PumpCart®, or vial | Refrigerated (36°F to 46°F) | Until expiration date | ||
| FlexTouch®, PenFill®, or vial | Room temperature (up to 86°F) | Up to 28 days | ||
| PumpCart® | Room temperature (up to 86°F) | Up to 18 days | ||
| After first use (opened) | Temperature | Use time | ||
| FlexTouch® or vial | Refrigerated (36°F to 46°F) — OR — Room temperature (up to 86°F) |
Up to 28 days | ||
| PenFill® | Room temperature (up to 86°F) | Up to 28 days | ||
| PumpCart® | Room temperature (up to 98.6°F) | Up to 4 days | ||
e3-mL PenFill® cartridge is available for NovoPen Echo®.
fDo not freeze Fiasp®. Do not use Fiasp® if it has been frozen.
gDo not refrigerate Fiasp® PenFill® or PumpCart® cartridges after first use.
PumpCart® may be kept at room temperature for a maximum of 18 daysh
Replace the Fiasp® PumpCart® cartridge at least every 4 days, or according to the pump user manual, whichever is shorter, even if you have not used all of the insulin. Do not refrigerate PumpCart® after first use.
hIncluding 4 days in the pump.
Here are a few tips for traveling with Fiasp®:

Pack extra
Bring extra supplies with you in case you are delayed, lose something, or decide to extend your trip.

Proper storage
Your insulin must be kept at room temperature (below 86°F), so be sure not to store it in places like the trunk or glove box of a car or checked baggage on a flight where the temperature may get too hot or cold.

Keep on hand
If you are flying, make sure that you take your insulin and other diabetes medicines with you in your carry-on bag. Checked luggage could get lost or delayed! The Transportation Security Administration (TSA) allows you to bring diabetes supplies in your carry‑on.

Communicate
It is a good idea to let TSA agents know before the security screening process begins that you have diabetes and are carrying supplies. You can continue to wear your insulin pump while going through a common airport metal detector as it will not harm the device or trigger an alarm. However, you should not send your pump device through an X-ray machine.
Where can I get help with the cost of Fiasp®?
If you have private or commercial insurance, such as insurance you receive through an employer, you can register for the Fiasp® Savings Offer. With the offer, you may be eligible to pay as little as $35 per 30-day supply (maximum savings of $65 per 30-day supply) or no more than $99 per prescription for up to 2 years when you start Fiasp®.i
Leading a healthier life with diabetes can be tough, but NovoCare® is here to help
NovoCare® offers bite-sized, everyday lessons on eating better, moving more, and treating diabetes that you can learn in 5 minutes or less. Sign up to receive emails with tips for leading a healthier life and updates about Fiasp®.

